Latent Heat is the energy contained in or released by a substance during temperature changes, or phase changes between solid, liquid, and gas.
A measure for this is the calorie, the energy needed to heat one gram of water 1°C. For comparison, a teaspoonful of water is about 5 grams, the weight of a nickel. The Calorie (note capitalization) used in dietary calculations is a unit of 1000 calories (small c).
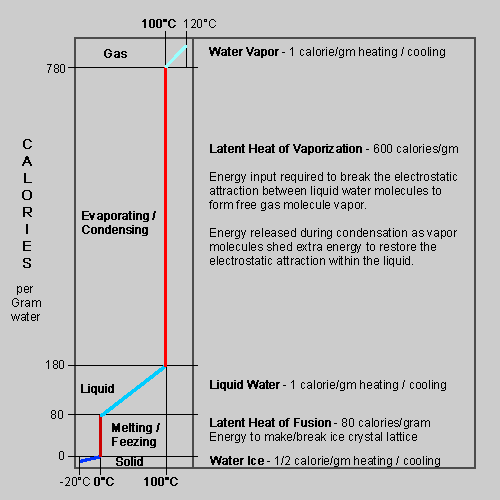
The energy going into the water to heat it or change phase is returned to the envrionment when the water is cooling or changing back. From the chart on the right, steam (vapor) condensing from 100°C gas to 100°C liquid releases 600 cal/g, compared to only 100 cal/g to drop the 100 degrees to 0°C!
|
Latent Heat of:
|
Calories/Gram
|
|
Gas
|
1.0
|
|
Vaporization
(Evaporate/Condense) |
600
|
|
Liquid
|
1.0
|
|
Fusion
(Melt/Freeze) |
80
|
|
Ice
|
0.5
|
|
Sublimation
(Evaporate/Deposit) |
780
|
thunderstorms, steam engines, and injuries from steam burns.
The physics (math alert!) behind all this is shown here.
The Skew-T accounts for this energy gain or release as parcels change altitude by the Dry and Moist Adiabats.
Supercooling, the condition where liquid water exists below freezing, is common in the atmosphere down to about -22°C due the effects of latent heat. This directly affects the limits of Icing and formation of Thunderstorms. Note that the Latent Heat of Fusion is about 7% of the Heat of Vaporization. This means there is another hurdle for the cooling parcel to overcome to change from liquid to ice. Three problems add to the delay:
- Parcels trying to shed heat through radiation (diabatic cooling) are surrounded by other parcels trying the same thing. This can make the interior of a rising cloud mass significantly warmer than the adiabatic evironment outside the cloud. (Cloud edge evaporation adds to this, but that's a different story.)
- Water droplets in the parcel have formed on condensation nuclei, usually dust and salt, which dissolve and lower the freezing temperature for that droplet.
- The water ice crystal structure is easier to form once some ice is present. Brownian motion within the droplet disrupts crystal formation until the random energy variations drop enough to allow a seed crystal to form somewhere within the droplet. The difference in surface tension between the ice crystal and droplet surface then permit rapid lattice building onto the seed. This can then quickly spread to other liquid droplets as fast as Heat of Fusion can be shed.
These effects can be seen in Altostratus changing to Cirrus, and in aircraft contrails. The best example is Cumulonimbus Type 3 transitioning to Cumulonimbus Type 9. The sharp, cauliflower cloud top edges are from supercooled liquid droplets in the CB3 updraft. The fuzzy edges over top and edges of the CB9 Anvil are from the ice crystal formation, all because of latent heat.